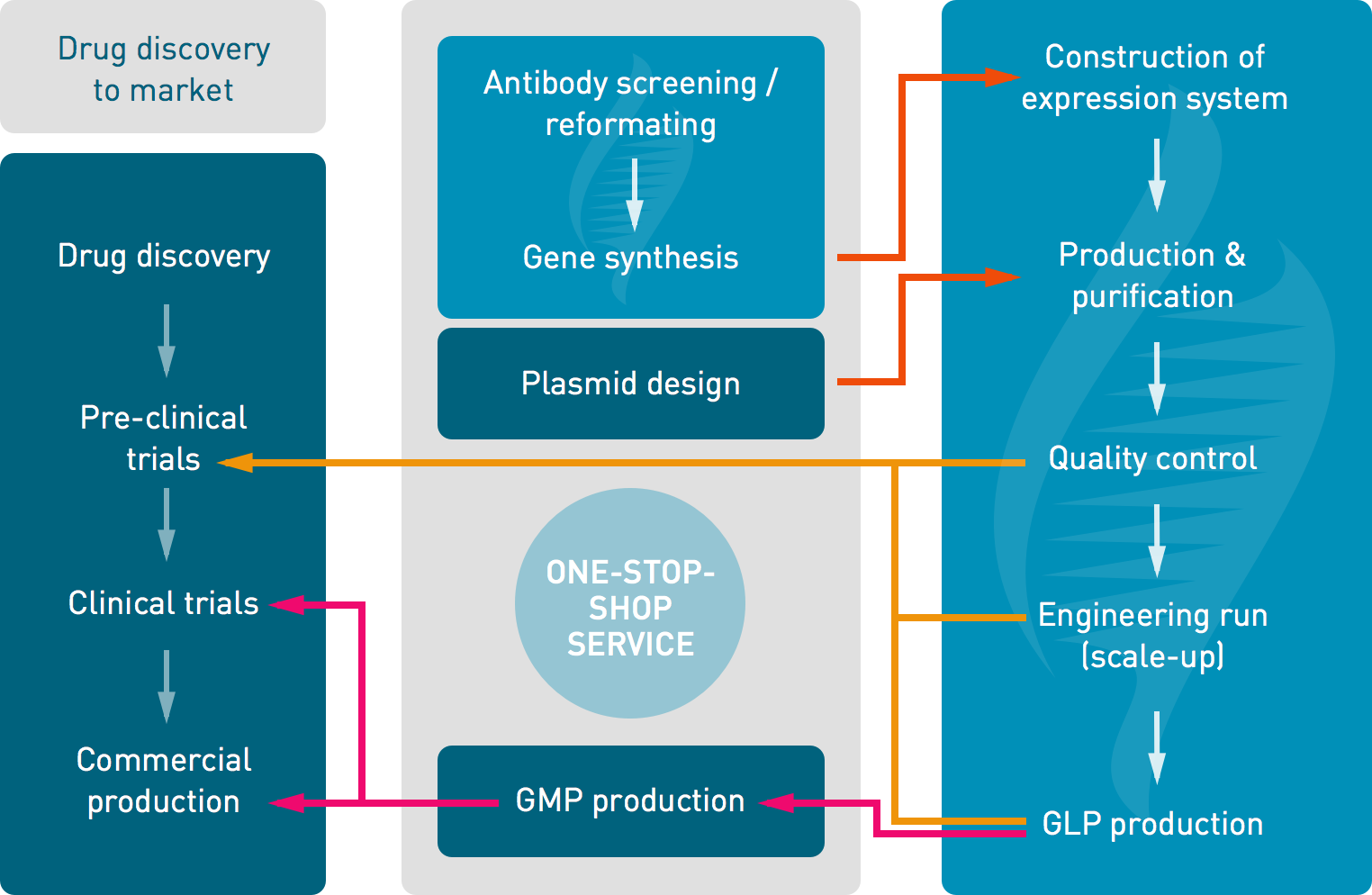
Xpress Biologics ensures a “one-stop-shop” service from the design of expression systems to the management of clinical trials. It encompasses the following activities:
- consultancy, project set-up, risk assessment, regulatory support, project management
- development of expression systems and of production and purification methods
- production of biologics
- scaling-up studies and industrialization
Example of the “one-stop-shop” service proposed for the production of antibody fragments:
- antibody screening and reformatting
- DNA optimization, gene synthesis, cloning in specific vectors and transformation in P. pastoris
- “R&D” grade production, purification and quality controls using “off-the-shelf” methods for in vitro trials
- process optimization, scaling-up studies and full QC development
- GLP grade production, purification and quality controls
- management of pre-clinical trials
- transfer to a manufacturing company for GMP grade production
- management of clinical trials